What’s the deal with silver fillings? Why is it that we see news stories every once in awhile questioning the safety of dental amalgam? The answer, in a nutshell, is Mercury. 
The element Mercury (Hg) is highly toxic. Think broken thermometers and fluorescent light bulbs. Avoiding Mercury exposure is highly recommended. As an element, Mercury is poisonous.
Another element, Chlorine (Cl), is also highly toxic. Yet, when combined with the element Sodium (Na) you get a chemical that is common, safe and in reasonable doses, delicious. Common table salt is a chemical compound called Sodium chloride (NaCl) and is perfectly safe to eat in moderate amounts. But no one in their right mind would go out of their way to ingest Sodium or Chlorine on their own. The same goes for Mercury.
“Silver fillings” aren’t really fillings made of Silver. They are a combination of Mercury, Silver, Copper, Tin and other trace metals. Silver fillings are placed by thoroughly mixing these ingredients. The ingredients mix and form an alloy of the metals. This alloy is different than any of the ingredients individually. In other words, there isn’t just Mercury, Silver, Tin or Copper in there. It’s a whole new chemical compound made up of all of these metals. It’s kind of like concrete. You start with cement, sand, stone and water. The final product is concrete. You can’t go back and take the ingredients out of concrete without breaking down the concrete chemically.
The bottom line is that there’s no such thing as “Mercury fillings.” Dental amalgam has Mercury in it that is chemically combined with other metals to form an alloy. One of the properties of Mercury is it’s ability to form an alloy like this at room temperature.
Can dental amalgam “leak” Mercury? Yes. There can be a very slight release of mercury from amalgam fillings. A study conducted by measuring the Mercury vapor levels inside the mouth over a 24-hour period in patients with at least nine amalgam restorations showed the average daily dose of inhaled mercury vapor was 1.7 µg (micrograms), which is approximately only 1% of the threshold limit value of 300 to 500 µg/day established by the World Health Organization. So there is Mercury released from fillings, but it’s a very tiny amount.
What about Mercury exposure from dental amalgams causing diseases? The American Dental Association has weighed in regarding the safety and efficacy of dental amalgam. Scientific evidence concludes that the use of dental amalgam is safe. There is no evidence to support removing silver fillings in an effort to cure or prevent other diseases.
Dental amalgam has undoubtedly saved millions of teeth in its 100+ years of use. Until relatively recently there haven’t been inexpensive options to restore teeth that could hold a candle to silver fillings. They’re durable as heck and they’re relatively easy and inexpensive to place.
Are there any problems with dental amalgam? I actually see two. 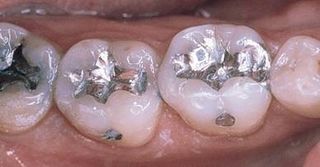
- They’re ugly. When polished they can be shiny and smooth, but they don’t look like a tooth. They look like metal, which they are.
- In order to place a silver filling you need to remove a lot of tooth structure. In a tooth that’s never been filled before, this means that you’re cutting away more tooth structure than you need to.
To me, those are the main down sides to using dental amalgam. Perhaps these down sides deserve their own post (stay tuned!) I place very few dental amalgams any more because I’m confident that I can place an excellent bonded resin restoration (a.k.a: composite) in any situation that I might have used amalgam.
But my reasons for using composite fillings has nothing to do with Mercury. In my mind, the Mercury is a non-issue.
Do you have questions or comments about this post? Can this Saginaw dentist be of service to you in any way? I’d love to hear from you at my email: alan@meadfamilydental.com. Or give us a call at the office at (989) 799-9133! We’d love to be your Saginaw dentist! And don’t forget to “like” us on Facebook!




awesome!